 AI
in Healthcare
AI
in Healthcare
History, Breakthroughs, and the Road Ahead
From penicillin to MRI machines to gene sequencing, American healthcare has always been shaped by technology. But no force has reshaped medical practice as profoundly as artificial intelligence, with the prospect of more to come in the future.
What began as fragile, experimental systems in the 1970s has grown into a powerful diagnostic engine, a research accelerator, and a companion to doctors and patients alike. AI in healthcare is not just a technological revolution; it is a transformation of how the U.S. understands disease, delivers care, and imagines the future of human wellness.
This article traces the journey from early symbolic reasoning systems to today's multimodal AI models, showing how each era of innovation pushed healthcare forward, and how America became a global leader in medical AI.
The integration of artificial intelligence into American healthcare represents one of the most dramatic technological shifts in modern medicine. From its humble beginnings in the 1970s to today's sophisticated deep learning systems, AI has evolved from a theoretical concept into a practical tool that touches nearly every aspect of patient care, medical research, and healthcare administration.
 Early History: The Pioneering Years
Early History: The Pioneering Years
AI entered American hospitals long before smartphones or the modern internet. The earliest systems were rule-based expert programs that were precursors to modern machine learning. The story of AI in American healthcare begins with the development of expert systems designed to mimic the diagnostic reasoning of physicians.
One of the first and most influential projects was MYCIN, developed at Stanford University between 1972 and 1980. This rule-based system could diagnose bacterial infections and recommend appropriate antibiotics with accuracy that often matched or exceeded that of human specialists. Though MYCIN never entered clinical practice due to technical limitations and regulatory concerns, it demonstrated the potential for computers to assist in complex medical decision-making.
The MYCIN expert system is one of the earliest and most influential rule-based expert systems developed in the field of artificial intelligence. It was created in the early 1970s at Stanford University by Edward Shortliffe under the supervision of Bruce Buchanan and others as part of a doctoral research project.
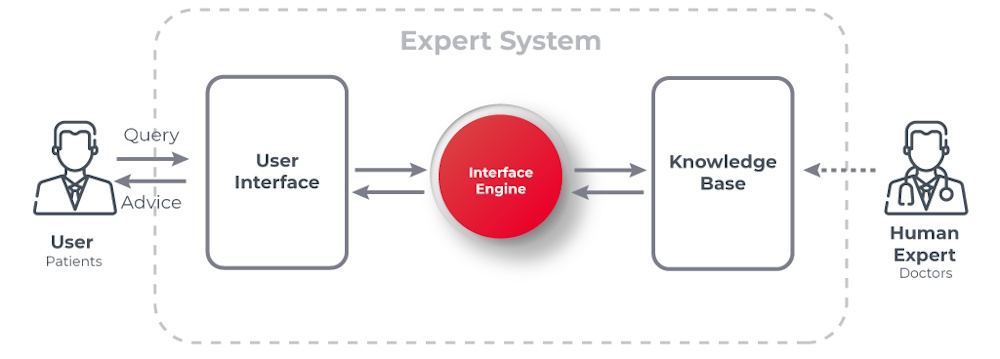
MYCIN was designed to diagnose bacterial infections (particularly bacteremia and meningitis) and recommend appropriate antibiotic treatments, including dosage adjustments based on the patient's body weight. MYCIN used around 600 production rules (if-then statements) encoded by human experts (infectious disease specialists).
For example:
IF the infection is primary-bacteremia
AND the site of
the culture is blood
AND the suspected portal of entry is the
gastrointestinal tract
THEN there is suggestive evidence (0.7) that the
infection is bacteroid.
MYCIN introduced a novel method for dealing with uncertainty using certainty factors (CF), ranging from -1 (definitely false) to +1 (definitely true). It combined evidence using ad hoc formulas to update belief in hypotheses. MYCIN used backward chaining. That is, starting from a hypothesis like "What bacteria is causing the infection?", it asked questions to gather evidence. It was capable of explaining its reasoning. Users could ask "Why?" or "How?" to understand recommendations. MYCIN separated domain knowledge (rules) from the inference engine, a foundational idea in expert systems (the so-called "knowledge is power" principle).
In tests, MYCIN's performance was comparable to or better than that of infectious disease specialists. However, it was never used in clinical practice due to a lack of integration with existing hospital systems. There were also legal and ethical concerns, especially the issue of liability for incorrect advice. And it was limited because it couldn't access real patient data directly.
A "shell" version of MYCIN called EMYCIN (Essential MYCIN) was created as a spinoff. It came without the medical knowledge, allowing other domains to build expert systems (PUFF for lung disorders, GUIDON for teaching) off of it. MYCIN inspired the development of other expert systems like PROSPECTOR (geology/mineral exploration). MYCIN helped establish expert systems as a major AI paradigm in the decade of the 70s, and pioneered explainable AI (XAI), long before the term existed. Around the same time as MYCIN, researchers at the University of Pittsburgh developed INTERNIST-I, an expert system designed to diagnose complex problems in internal medicine.
These early systems operated on knowledge bases containing hundreds or thousands of rules encoded by medical experts, representing the cutting edge of what was then possible with AI technology. However, these systems were limited by their brittleness, an inability to learn from new data, and the immense effort required to encode medical knowledge manually.
The 1980s and 1990s saw continued experimentation with medical expert systems, but progress was slow. The limitations of rule-based approaches became increasingly apparent, and the field entered what some called an "AI winter" for healthcare applications. Computers lacked the processing power needed for more sophisticated approaches, and the medical community remained skeptical about replacing human judgment with machines.
 The Modern AI Revolution in Healthcare
The Modern AI Revolution in Healthcare
The true transformation of AI in American healthcare began in the 2010s, driven by three key developments: the explosion of electronic health records providing vast amounts of data, dramatic increases in computing power particularly through graphics processing units, and breakthroughs in machine learning algorithms, especially deep learning neural networks.
The passage of the Health Information Technology for Economic and Clinical Health Act (HITECH Act) in 2009 accelerated the adoption of electronic health records across American hospitals and clinics. This created enormous datasets that could be used to train AI systems, fundamentally changing what was possible. Rather than relying on hand-coded rules, modern AI systems could learn patterns directly from millions of patient records, imaging studies, and clinical outcomes.
Today, AI is rapidly transforming the healthcare industry by improving diagnostic accuracy, reducing hospital readmissions, and automating administrative tasks in clinical settings. AI has the potential to improve outcomes by enabling faster and more precise diagnoses, personalizing treatment plans based on patient-specific data, and optimizing clinical workflows.
AI in healthcare leverages advanced technologies such as machine learning (ML), natural language processing (NLP), deep learning (DL), and predictive analytics, which support clinicians in decision-making and help deliver better care to patients.
AI is improving the healthcare industry by making clinical processes faster, more accurate, and more proactive. From early triage to long-term patient monitoring, AI streamlines multiple processes and also helps in R&D. Here are some of the dimensions of AI usage in healthcare:
- Pre-diagnosis: AI assists in initial assessment and prioritization.
- Treatment: AI supports treatment planning and care coordination.
- Administration: AI streamlines administrative, billing, and operational tasks.
- Diagnostics: AI aids in accurate and timely interpretation.
- Monitoring: AI enables remote monitoring and follow-up care.
Pre-diagnosis and triaging
Organizations are utilizing AI in healthcare for pre-diagnosis and triaging by assessing patient conditions early, leveraging the analysis of symptoms, medical history, and real-time data. Moreover, artificial intelligence in healthcare can also help prioritize cases based on patient condition. This process can reduce clinical workload, shorten waiting times, and ensure faster care for patients.
Treatment planning and care coordination
AI assists doctors in creating a personalized treatment plan for every patient, based on their health records. It also helps doctors in evaluating patient data, genetic markers, lifestyle factors, and disease progression. Additionally, AI ensures that all the teams stay coordinated for better outcomes, fewer complications, and more efficient care delivery.
Administrative, billing, and operations
AI automates the backend operations, including appointment booking, billing, and insurance claim management. It automates repetitive tasks, minimizes administrative mistakes, and helps accelerate reimbursements. In hospitals, AI is used to optimize the distribution of resources, schedule staff, and manage the supply chain to ensure that healthcare facilities operate at a higher level of efficiency and lower costs.
Diagnostics and clinical interpretation
AI-powered diagnostic systems enhance accuracy by identifying complex patterns that humans cannot detect. In addition, they also analyze X-rays, MRIs, and CT scans to interpret lab reports and pathology slides. This system can help improve clinical decision-making and accelerate the diagnostic process.
Remote monitoring and follow-up care
With the implementation of AI in the healthcare system, doctors can continuously monitor their patients without visiting them. They can do this through wearables, sensors, and IoT-based health devices. These systems track patient conditions like vital signs, detect anomalies, and alert clinicians before the conditions worsen.
 Core AI Technologies Used in Healthcare
Core AI Technologies Used in Healthcare
AI in healthcare is powered by multiple, underlying AI technologies such as machine learning (ML), natural language processing (NLP), computer vision, and automation frameworks.
Machine Learning and Predictive Analytics
Machine learning in healthcare plays a vital role, as it can analyze a vast amount of data and genomics information to identify patterns, build models, and forecast possible outcomes. With the use of predictive analytics, events like disease progression or readmissions can be anticipated. In addition, integrating machine learning and predictive analysis technologies into the healthcare system helps manage chronic diseases at an early stage. It flags high-risk patients early so that clinicians can design an effective treatment plan tailored to their individual needs.
Natural Language Processing (NLP)
NLP works by analyzing unstructured text from clinical notes, reports, and patient interactions using techniques like tokenization and sentiment analysis to extract meaningful insights. NLP also helps in automating the summarization of medical records and supports clinical decision-making. NLP enables AI chatbots in healthcare to understand and answer patient queries. This feature enables doctor-patient interaction without requiring an in-person visit and saves time for both doctor and patient. Even for common illnesses like coughs and mild infections, patients can consult doctors online and receive the right medication and guidance at home.
Computer Vision
The role of computer vision in the healthcare industry is to analyze medical images (X-rays, MRIs, and CTs) for faster, more accurate disease detection. It also helps in detecting anomalies, segmenting tissues, and classifying conditions through pixel-level pattern recognition. Computer vision aids in diagnostics in radiology by identifying tumors or fractures faster than can be done by human review alone. This AI technology helps in surgical planning with 3D reconstructions and supports remote monitoring via endoscopy analysis.
Robotic Process Automation (RPA)
Of all the AI technologies used in healthcare, RPA is one of the most in-demand. This is because it helps in automating regular tasks, saves valuable time, and streamlines tasks like data entry, claims processing, and appointment scheduling via screen scraping and API integrations. Moreover, it supports revenue cycle management, verifies insurance eligibility, and manages inventory to minimize human intervention. Hospitals facing staffing shortages can benefit from RPA to automate routine operational workflows, reducing errors and easing the burden on clinical and administrative staff.
Voice Recognition
Voice recognition is another one of the high-demand AI-powered technologies. This is because it converts spoken language into text using acoustic models and deep learning to transcribe consultations accurately. As one of the core applications of AI, voice recognition and virtual assistants assist in real-time scribing, enable hands-free EHR updates, and triage symptoms. As a result, it minimizes the documentation time and provides higher patient engagement.
 Current Applications
Current Applications
Medical imaging has become one of the most successful application areas for AI in American healthcare. Deep learning algorithms can now analyze X-rays, CT scans, MRIs, and pathology slides with remarkable accuracy. The FDA has approved numerous AI-based imaging devices that can detect diabetic retinopathy in eye scans, identify suspicious lesions in mammograms, and flag potential strokes in brain scans. These systems don't replace radiologists but serve as powerful assistive tools, helping to prioritize urgent cases and reduce diagnostic errors.
In radiology departments across America, AI algorithms work alongside human experts, sometimes analyzing images faster than they can be formally interpreted. Studies have shown that combining human expertise with AI often produces better results than either working alone, demonstrating the complementary nature of human and machine intelligence in medicine.
Predictive analytics represents another major application domain. Hospitals use AI systems to predict which patients are at highest risk for complications like sepsis, heart failure, or hospital readmission. These early warning systems analyze continuous streams of data from electronic health records, vital signs monitors, and laboratory results to identify subtle patterns that might escape human notice. When a patient's risk score crosses a threshold, the system alerts clinicians who can intervene before a crisis develops.
The COVID-19 pandemic accelerated AI adoption in several areas. Healthcare systems deployed AI-powered tools to predict patient deterioration, optimize resource allocation, and identify high-risk populations. Some hospitals used machine learning algorithms to help determine which patients needed ICU care or mechanical ventilation, decisions that became critical when resources were scarce.
Drug discovery and development have also been transformed by AI. American pharmaceutical companies and research institutions now routinely use machine learning to identify promising drug candidates, predict how molecules will interact with biological targets, and optimize clinical trial designs. AI systems can screen millions of potential compounds in silico, dramatically reducing the time and cost of early-stage drug development. Several drugs discovered with the assistance of AI are currently in clinical trials, representing a new paradigm in pharmaceutical research.
 The Role of AI in Drug Discovery
The Role of AI in Drug Discovery
The journey from identifying a potential therapeutic compound to delivering a safe, effective drug to patients is a notoriously long, complex, and expensive process. Traditionally, drug discovery has been likened to finding a needle in a haystack, a laborious process involving trial and error, extensive laboratory work, and years of clinical trials.
But today, a technological revolution is reshaping the pharmaceutical world. At the forefront of this transformation stands artificial intelligence. It is a force that is accelerating innovation, improving precision, and bringing us closer to a future where personalized, affordable, and efficient medicine is no longer a dream but a reality.
AI is now deeply entrenched in real-world healthcare challenges. From pattern recognition and predictive modeling to data mining and molecular simulation, AI is becoming the ultimate lab assistant, for it is able to sift through oceans of data, propose hypotheses, and design experiments in silico with unprecedented speed. In the high-stakes world of drug development, where time can mean the difference between life and death, AI is emerging as an indispensable ally.
The Traditional Drug Discovery Pipeline
Before we can appreciate the impact of AI, it's important to understand what it's replacing or enhancing.
The traditional drug discovery pipeline is a multistep journey that typically spans 10 to 15 years and costs upwards of $2.6 billion. It begins with basic research, identifying disease mechanisms and potential drug targets; usually proteins or genes involved in disease pathways.
Next comes lead compound discovery, where libraries of molecules are screened for those that might interact with the target. Promising candidates are optimized for safety, efficacy, and pharmacokinetics. This is followed by preclinical testing in lab animals and then clinical trials in human volunteers, divided into three rigorous phases.
Unfortunately, most drug candidates fail along the way. Fewer than 10% of drugs entering clinical trials reach the market. Failures often stem from poor efficacy, unexpected toxicity, or flawed clinical trial design. The high attrition rate and ballooning costs have made the pharmaceutical industry ripe for disruption.
This is where AI steps in; not just as an enhancement but as a revolutionary force capable of redesigning the entire process.
Target Identification: AI Illuminates the Invisible
The first step in drug development - identifying a suitable molecular target - is like hunting in the dark. The human body has tens of thousands of genes and proteins, and pinpointing the one responsible for a particular disease can be overwhelming.
AI algorithms, particularly machine learning and deep learning systems, excel at analyzing massive datasets; genomic sequences, transcriptomic profiles, proteomics, and clinical data. By training on known disease-gene interactions, AI models can uncover hidden patterns, predict novel targets, and prioritize them based on biological relevance and druggability.
For example, natural language processing (NLP) algorithms can scan millions of scientific articles, patents, and clinical records to unearth relationships between genes and diseases. Graph-based neural networks can map complex biological pathways and reveal critical nodes worth targeting. With AI, researchers can move from intuition-driven guesswork to data-driven precision.
Molecular Design and Compound Screening
Once a target is identified, the next challenge is finding a molecule that can interact with it effectively. This is usually done by binding to the protein and modulating its function. Traditionally, this required physical screening of millions of compounds, which is an expensive and time-consuming process.
AI has dramatically changed the game. Virtual screening powered by AI allows researchers to model the target in three dimensions and predict how different molecules will bind to it. Deep learning models trained on databases of chemical structures and bioactivity data can generate novel molecules with desired properties, an approach known as de novo drug design.
Generative adversarial networks (GANs), variational autoencoders, and reinforcement learning models are now capable of creating entirely new compounds, optimizing them for binding affinity, solubility, toxicity, and other pharmacokinetic parameters. These AI-designed molecules can be synthesized and tested far more rapidly than those discovered by conventional means.
A landmark example occurred when Insilico Medicine used AI to design a novel drug candidate for idiopathic pulmonary fibrosis in just 46 days. This was an astounding feat compared to traditional timelines.
Predicting Pharmacokinetics and Toxicity:
The success of a drug depends not only on its efficacy, but also on its behavior in the human body; how it's absorbed, distributed, metabolized, and excreted (ADME). A promising molecule might be rendered useless if it degrades too quickly, accumulates in the wrong tissue, or produces harmful byproducts.
AI models, trained on pharmacokinetic and toxicity data, can predict these outcomes with increasing accuracy. Support vector machines, random forests, and neural networks can assess the likelihood that a compound will be metabolized by certain enzymes, cross the blood-brain barrier, or cause liver damage.
By flagging red flags early in development, AI helps reduce late-stage failures, thus saving time, money, and lives. This predictive power is especially valuable in toxicology, where traditional animal models are expensive, time-consuming, and often fail to predict human outcomes.
Preclinical Testing: Virtual Labs and Simulated Biology
In the preclinical phase, drug candidates are tested in cell cultures and animals to assess efficacy and safety. This stage is critical but fraught with ethical and practical concerns. Animal models don't always reflect human biology, and experiments can be slow and costly.
AI is beginning to offer alternatives. Computer simulations known as in silico models can replicate biological systems at multiple scales, from molecular interactions to organ-level physiology. Digital twins, virtual avatars of patients or animals, can be used to simulate drug effects under various conditions.
Machine learning can also help analyze data from lab experiments by detecting subtle patterns, optimizing protocols, and even controlling robotic lab systems. Combining AI with automation, researchers can perform high-throughput screening, analyze results in real-time, and make data-driven decisions on which compounds to advance.
Clinical Trials: Smarter Design, Better Outcomes
Clinical trials are the most expensive and time-consuming part of drug development. They require recruiting diverse participants, designing appropriate protocols, monitoring outcomes, and ensuring compliance, all while dealing with the unpredictability of human biology.
AI is bringing intelligence to this complexity. By analyzing electronic health records, genomic data, and social determinants of health, AI can help identify suitable participants matching them to trials based on detailed criteria. This not only accelerates recruitment but also improves representation and reduces bias.
Predictive models can optimize trial design by suggesting dosage levels, endpoints, and patient stratification strategies. AI can monitor patient adherence, flag adverse events, and detect patterns that would be invisible to human analysts.
Companies like Deep 6 AI and Unlearn.ai are already using machine learning to create synthetic control arms. These are digital cohorts that reduce the need for placebo groups by simulating their outcomes. This ethical innovation could transform how we test new drugs, especially in rare or life-threatening conditions.
Post-Approval and Real-World Data
Even after a drug reaches the market, the story isn't over. Real-world use often reveals side effects, interactions, or population-specific responses not seen in trials. AI is playing a vital role in post-marketing surveillance, pharmacovigilance, and real-world evidence (RWE) generation.
Natural language processing systems can analyze adverse event reports, social media posts, and medical literature to detect early warning signs. Machine learning can track prescription patterns, hospitalizations, and biomarker data to assess long-term safety and effectiveness. This feedback loop not only protects patients but also informs future drug development. By learning from every success and failure, AI systems become smarter, more accurate, and more impactful over time.
Challenges and Limitations
While AI holds immense promise, it's not a silver bullet. There are significant challenges that must be addressed before AI can fully realize its potential in drug discovery.
Data quality remains a major hurdle. AI models are only as good as the data they're trained on. Inconsistent, biased, or incomplete data can lead to faulty predictions. Access to proprietary pharmaceutical data is also limited, which can hamper model training and validation.
Transparency is another issue. Many AI models, especially deep learning systems, function as "black boxes," making it hard to understand how they arrive at their conclusions. This lack of explainability can hinder regulatory approval and erode trust among clinicians and researchers.
Regulatory frameworks are still evolving. Agencies like the FDA and EMA are working to establish guidelines for AI-based tools, but questions remain about validation, accountability, and ethical use. Ensuring patient privacy, avoiding algorithmic bias, and maintaining scientific rigor are critical priorities.
Finally, the integration of AI into existing workflows requires cultural change, interdisciplinary collaboration, and robust infrastructure. Scientists, engineers, regulators, and ethicists must work together to harness AI responsibly and effectively.
Trust, Transparency, and Human Oversight
AI in drug development raises profound ethical questions. Can we trust algorithms to make decisions that affect human lives? How do we ensure that AI systems don't perpetuate or amplify biases present in historical data? What safeguards are needed to prevent misuse or unintended consequences?
Transparency and explainability are key. Researchers are developing techniques like SHAP values and LIME to interpret model predictions. Regulations are pushing for algorithmic accountability and patient consent.
Human oversight is essential. AI should augment, not replace, expert judgment. The goal is not to hand over drug discovery to machines, but to create a synergistic relationship where humans and AI collaborate, combining intuition, creativity, and compassion with computational power and data-driven insight.
Success Stories and Case Studies
Numerous companies and research institutions are already demonstrating the real-world power of AI in drug discovery.
- Atomwise uses deep learning to predict how small molecules interact with proteins. Its AtomNet platform has identified promising candidates for diseases like Ebola, multiple sclerosis, and leukemia.
- BenevolentAI integrates scientific literature, clinical data, and genomics to discover new drug targets. In 2020, it identified baricitinib - a rheumatoid arthritis drug - as a potential treatment for COVID-19, a finding later validated in clinical trials.
- Exscientia uses AI to automate the entire drug design process. In 2021, it became the first company to put an AI-designed molecule into human trials. Their approach reduces the average timeline from four years to less than 12 months.
These examples are just the beginning. Hundreds of startups, academic labs, and pharmaceutical giants are investing in AI, signaling a tectonic shift in how we discover and develop medicines.
Toward a Smarter, Faster, Healthier World
As AI continues to evolve, its role in drug discovery will only deepen. Future systems may integrate quantum computing, multi-omics data, and real-time clinical feedback to create personalized drugs on demand. Digital twins of individual patients could be used to simulate responses to treatments before they're administered.
Drug repurposing (finding new uses for existing drugs) will be turbocharged by AI's ability to match molecular mechanisms with disease pathways. Precision medicine, where treatments are tailored to a patient's genetic and environmental profile, will become the norm rather than the exception.
Ultimately, AI has the potential to democratize drug development. By lowering costs, reducing timelines, and increasing accessibility, AI could help deliver life-saving therapies to populations that have long been underserved.
The Convergence of Intelligence and Innovation
In the age-old battle against disease, artificial intelligence is emerging not as a distant observer but as an active participant, a partner in innovation, discovery, and healing. From unraveling the mysteries of biology to designing the medicines of tomorrow, AI is helping scientists dream bigger, move faster, and aim higher.
But with this power comes responsibility. The path forward requires not only technological excellence but ethical wisdom, collaborative spirit, and an unwavering commitment to patient well-being.
The role of AI in drug discovery and development is not just a story of machines. It's a story of possibility, of reimagining what medicine can be. And as we stand on the threshold of this new frontier, one thing is clear: the future of drug discovery is not just intelligent, it's extraordinary.
 Administrative Applications
Administrative Applications
Beyond direct patient care, AI has found extensive applications in healthcare administration and operations. Natural language processing systems help extract information from unstructured clinical notes, coding medical records for billing purposes, and identifying quality improvement opportunities. These systems can process documentation faster and more consistently than human coders, though they still require human oversight.
Revenue cycle management, the complex process of billing and collecting payment for healthcare services, has been streamlined through AI. Machine learning algorithms can predict which claims are likely to be denied, optimize billing codes, and identify patterns of fraud or abuse. Given that administrative costs account for a substantial portion of American healthcare spending, these efficiency gains are significant.
Chatbots and virtual health assistants have become increasingly common in American healthcare systems. These AI-powered tools can answer basic health questions, schedule appointments, provide medication reminders, and triage patients to appropriate levels of care. While still limited in their capabilities, these systems can improve access to healthcare information and reduce the burden on clinical staff for routine inquiries.
 Challenges and Concerns
Challenges and Concerns
Despite its promise, AI in American healthcare faces substantial challenges. Algorithmic bias represents a critical concern, as AI systems trained on historical data can perpetuate or even amplify existing healthcare disparities.
Studies have documented cases where AI algorithms performed less accurately for minority populations or systematically disadvantaged certain demographic groups. Addressing these biases requires diverse training data, careful algorithm design, and ongoing monitoring of system performance across different populations.
Data privacy and security present ongoing challenges. Healthcare AI systems require access to sensitive patient information, raising concerns about unauthorized access, data breaches, and inappropriate use of medical records. The Health Insurance Portability and Accountability Act provides a framework for protecting patient privacy, but applying these regulations to AI systems involves complex technical and legal questions that continue to evolve.
The question of liability remains unsettled when AI systems are involved in medical errors or adverse outcomes. If an AI algorithm misses a cancer diagnosis or recommends an inappropriate treatment, who bears responsibility? These questions have implications for medical malpractice law, professional liability insurance, and the willingness of healthcare providers to adopt AI tools.
Integration with existing healthcare workflows poses practical challenges. Many AI systems are developed in research settings and struggle to function effectively in the messy reality of clinical practice. Electronic health record systems vary widely, data quality can be inconsistent, and clinical workflows differ between institutions. Successfully deploying AI requires not just technical excellence but also careful attention to the human and organizational factors that determine whether a technology will be adopted and used effectively.
 Regulatory Landscape
Regulatory Landscape
The FDA has adapted its regulatory framework to address AI-based medical devices, but this remains a work in progress.
Traditional medical device regulation assumed that devices were relatively static, but AI systems can continuously learn and update their algorithms. The FDA has proposed new approaches for regulating these "adaptive AI" systems, but many questions remain about how to ensure safety and effectiveness while allowing beneficial innovation.
Some argue that current regulations are too slow and burdensome, stifling innovation in a rapidly evolving field. Others contend that AI systems pose unique risks and require even more rigorous oversight than traditional medical devices. Finding the right balance between innovation and safety remains a central challenge for regulators, industry, and the medical community.
 The Future Landscape
The Future Landscape
Looking forward, AI is likely to become increasingly integrated into virtually every aspect of American healthcare.
Personalized medicine, where treatments are tailored to individual patients based on their genetic makeup, lifestyle, and environmental factors, will increasingly rely on AI to make sense of complex multidimensional data. AI systems may help design personalized treatment plans for cancer patients, predict which individuals will respond to specific medications, and identify optimal prevention strategies based on personal risk factors.
The integration of AI with other emerging technologies promises new capabilities. Combining AI with wearable sensors and continuous monitoring devices could enable truly proactive healthcare, where problems are identified and addressed before they become serious. AI-powered surgical robots may perform complex procedures with superhuman precision, while augmented reality systems could guide surgeons through operations with real-time AI assistance.
However, realizing this potential will require addressing fundamental questions about the role of AI in medicine. How much autonomy should AI systems have in clinical decision-making? How do we ensure that AI enhances rather than replaces the human relationships that are central to healthcare? How do we distribute the benefits of AI equitably across diverse populations and communities?
 Conclusion
Conclusion
The history of AI in American healthcare reflects broader patterns in the development of AI as a field, from early optimism through disappointment to renewed success driven by new technologies and approaches. Today's AI systems are powerful tools that are already improving diagnostic accuracy, enabling earlier interventions, accelerating drug discovery, and streamlining healthcare operations.
Yet we remain in the early stages of this transformation. The AI systems currently in use, while impressive, are narrow in their capabilities and require substantial human oversight. The path forward involves not just technical advancement but also careful attention to ethics, equity, regulation, and the human dimensions of healthcare. The goal is not to replace human clinicians but to augment their capabilities, allowing them to provide better care to more patients while preserving the essential human elements of medicine.
As AI continues to evolve, its impact on American healthcare will likely be profound, touching everything from how diseases are diagnosed and treated to how healthcare systems are organized and financed. The challenge for the healthcare community, policymakers, and society is to guide this transformation in ways that maximize benefits while minimizing risks, ensuring that AI serves the fundamental goal of improving human health and wellbeing for all Americans.
 Links
Links
telefonicatech.com/en/blog/mycin-the-beginning-of-artificial-intelligence-in-medicine
geeksforgeeks.org/artificial-intelligence/expert-systems/
en.wikipedia.org/wiki/Expert_system
youtube.com/shorts/BHKJI9ZFu3Y
scribd.com/doc/89226318/Case-Study-of-Mycin
people.dbmi.columbia.edu/~ehs7001/Buchanan-Shortliffe-1984/Chapter-05.pdf
scribd.com/document/827100656/Case-Study-AI-149-1
people.dbmi.columbia.edu/~ehs7001/Buchanan-Shortliffe-1984/Chapter-32.pdf
http://watson.latech.edu/book/intelligence/intelligenceOverview4.html
pmc.ncbi.nlm.nih.gov/articles/PMC10769497/
indiaai.gov.in/article/exploring-mycin-an-early-backward-chaining-expert-system
pmc.ncbi.nlm.nih.gov/articles/PMC6697545/